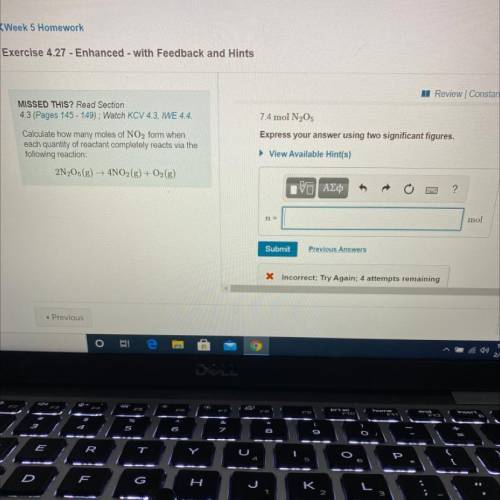
Calculate how many moles of NO2 form when
each quantity of reactant completely reacts via the
...

Chemistry, 23.02.2021 09:30 22MadisonT
Calculate how many moles of NO2 form when
each quantity of reactant completely reacts via the
following reaction:
2N205 (g) → 4NO2(g) + O2(g)
7.4 mol N2O5
Express your answer using two significant figures.
Will give brainliest! :)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
Questions

Mathematics, 24.11.2020 20:30



Mathematics, 24.11.2020 20:30


Mathematics, 24.11.2020 20:30

English, 24.11.2020 20:30


Mathematics, 24.11.2020 20:30


Mathematics, 24.11.2020 20:30



Mathematics, 24.11.2020 20:30


History, 24.11.2020 20:30


English, 24.11.2020 20:30





