
Chemistry, 23.02.2021 14:20 sadieanngraham15
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) concentration of acid is used at 1.5M
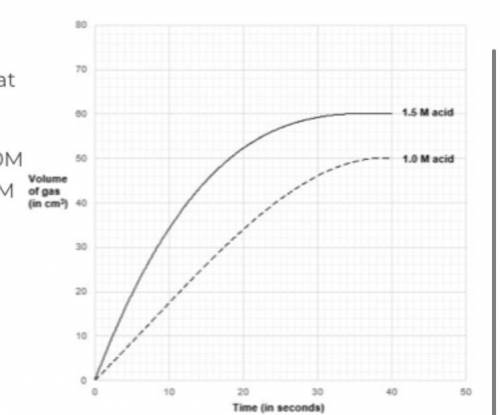

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) conc...
Questions



Mathematics, 12.05.2021 16:10

Mathematics, 12.05.2021 16:10




Mathematics, 12.05.2021 16:10





Social Studies, 12.05.2021 16:10



Mathematics, 12.05.2021 16:10

English, 12.05.2021 16:10

Mathematics, 12.05.2021 16:10

Advanced Placement (AP), 12.05.2021 16:10




