Please help I hope you can see the question
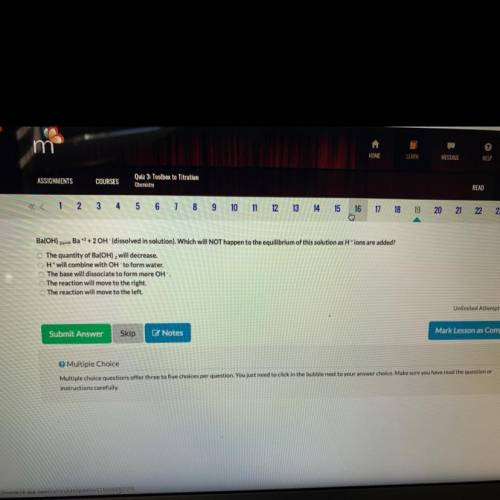
A. The quantity of ba(OH)2 will decrease
B. H+ w...

Chemistry, 23.02.2021 19:20 youcandoit13
Please help I hope you can see the question
A. The quantity of ba(OH)2 will decrease
B. H+ will combine with OH to form water
C. The base will dissociate to form more OH
D. The reaction will move to the right
E. The reaction will move to the left

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Questions

Health, 19.09.2019 07:30

History, 19.09.2019 07:30


Physics, 19.09.2019 07:30

Mathematics, 19.09.2019 07:30

Biology, 19.09.2019 07:30

English, 19.09.2019 07:30


History, 19.09.2019 07:30


Social Studies, 19.09.2019 07:30







Mathematics, 19.09.2019 07:30




