Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1


Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
You know the right answer?
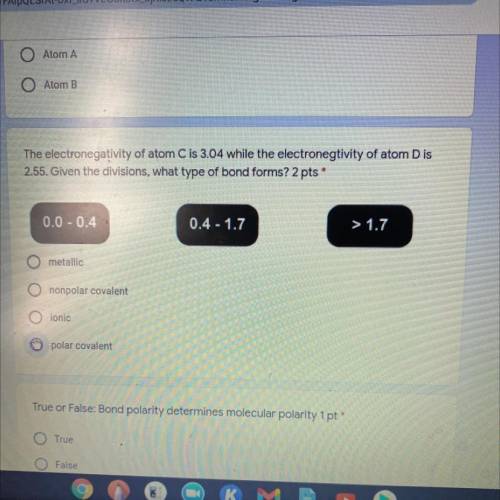
The electronegativity of atom C is 3.04 while the electronegtivity of atom D is
2.55. Given the div...
Questions

History, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01


Mathematics, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01












Mathematics, 17.10.2020 06:01

Biology, 17.10.2020 06:01

Mathematics, 17.10.2020 06:01