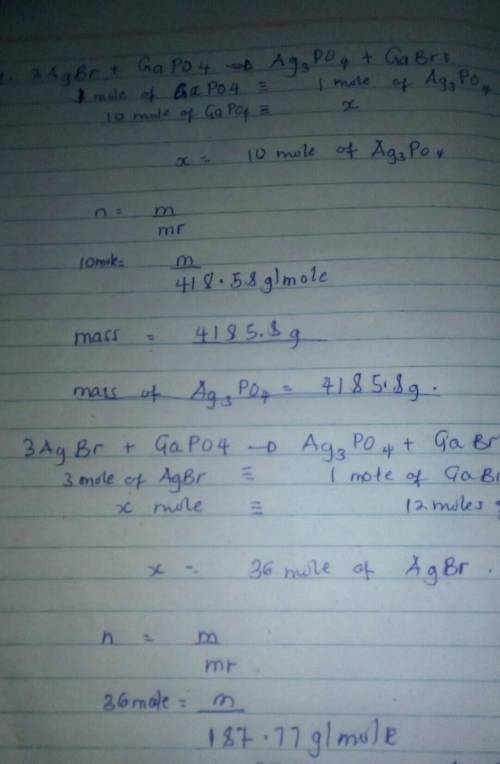Chemistry, 23.02.2021 21:20 aljalloh94
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles of GaPO4?
2. How many grams of AgBr are needed to produce 12 moles of GaBr3?
3. How many grams of GaBr3 are produced from the reaction of 15 moles of GaPO4?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles...
Questions