Question 8 (2 points)
3
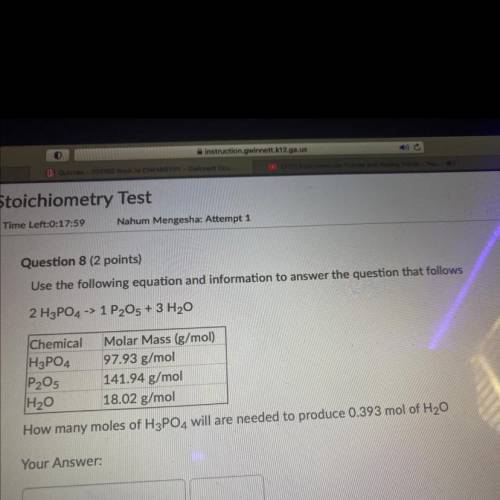
Use the following equation and information to answer the question tha...

Chemistry, 23.02.2021 22:10 msmaporter
Question 8 (2 points)
3
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P2O5 + 3 H2O
6
9
Chemical Molar Mass (g/mol)
H3PO4 97.93 g/mol
P2O5 141.94 g/mol
H20 18.02 g/mol
How many moles of H3PO4 will are needed to produce 0.393 mol of H20
Your
Answer
units

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Questions

Mathematics, 28.06.2019 01:30






Mathematics, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30


Mathematics, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30



Geography, 28.06.2019 01:30

Chemistry, 28.06.2019 01:30

Arts, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30

History, 28.06.2019 01:30

Mathematics, 28.06.2019 01:30




