
Chemistry, 23.02.2021 22:30 arnold2619
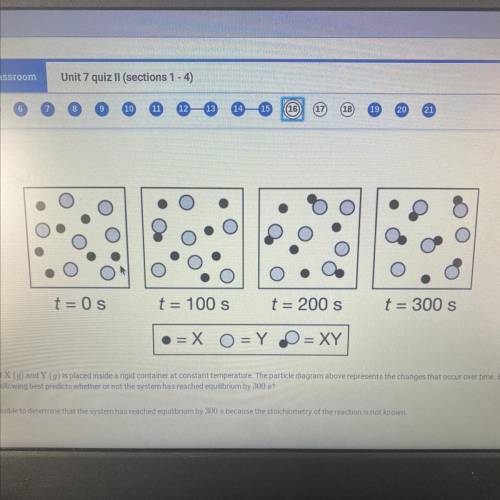
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature. The particle diagram above represents the changes that occur over time. Based on the particle
diagram, which of the following best predicts whether or not the system has reached equilibrium by 300 s?
a. It is not possible to determine that the system has reached equilibrium by 300 s because the stoichiometry of the reaction is not known.
b. it is not possible to determine that the system has reached equilibrium by 300 s because the amounts of X, Y, and XY have continued to change
c. The system has reached equilibrium by 300 s because the rate of formation of XY is constant
d. The system has reached equilibrium by 300 s because the rates of consumption of X and Y are equal

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3
You know the right answer?
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature....
Questions


Biology, 24.09.2019 16:30

Social Studies, 24.09.2019 16:30




Geography, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30

English, 24.09.2019 16:30

Mathematics, 24.09.2019 16:30

Social Studies, 24.09.2019 16:30


Mathematics, 24.09.2019 16:30




Biology, 24.09.2019 16:30


Mathematics, 24.09.2019 16:50



