
Chemistry, 23.11.2019 06:31 Jennifer16253
To completely neutralize a 0.325 g sample of pure aspirin, 15.50 ml of a sodium hydroxide solution is added. if 16.25 ml of the same sodium hydroxide solution must be added to an aspirin tablet sample during a titration to reach the endpoint, calculate the mass of aspirin in the table
a. 0.310 g
b. 0.288 g
c. 0.392 g
d. 0.450 g
e. 0.341 g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
To completely neutralize a 0.325 g sample of pure aspirin, 15.50 ml of a sodium hydroxide solution i...
Questions

English, 25.09.2019 20:30

Mathematics, 25.09.2019 20:30

Chemistry, 25.09.2019 20:30


Mathematics, 25.09.2019 20:30



English, 25.09.2019 20:30


Social Studies, 25.09.2019 20:30

Chemistry, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30


Social Studies, 25.09.2019 20:30

Social Studies, 25.09.2019 20:30



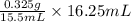 = 0.341 g
= 0.341 g


