
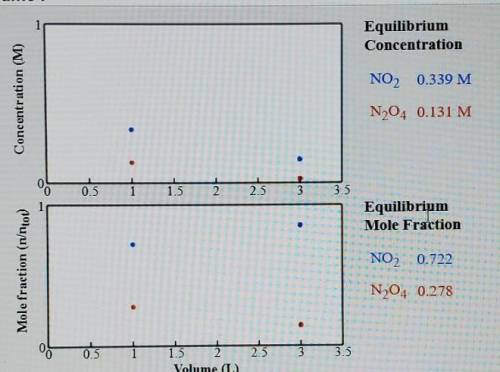
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) present in the container of each of these volumes.
K=0.880 if this is even needed.
I would appreciate just answering the 1.0L with steps so I can work out the second one. Thank you.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) pres...
Questions

Law, 13.11.2019 07:31


English, 13.11.2019 07:31

Chemistry, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31




Biology, 13.11.2019 07:31








Mathematics, 13.11.2019 07:31




