
Chemistry, 24.02.2021 05:50 Beast3dgar
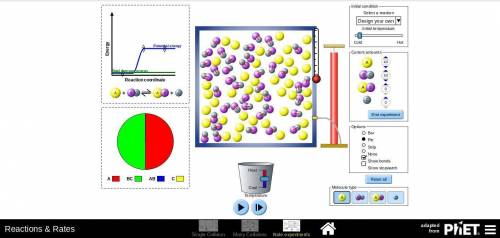
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen could be changed to alter the equilibrium. Use the simulation to test those changes. Describe how you used the simulation to model the changes and the results they produced. Use these methods if you find them helpful:
Look at the pie graph to see how the system changes.
Use the Temperature slider at the bottom to cool or heat the mixture.
Click the pause button on the simulation to observe the number of particles at any point of time.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

You know the right answer?
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen...
Questions

History, 18.10.2020 22:01




Mathematics, 18.10.2020 22:01

Chemistry, 18.10.2020 22:01

Computers and Technology, 18.10.2020 22:01


Mathematics, 18.10.2020 22:01


Chemistry, 18.10.2020 22:01


Biology, 18.10.2020 22:01

English, 18.10.2020 22:01

Mathematics, 18.10.2020 23:01


Biology, 18.10.2020 23:01

Physics, 18.10.2020 23:01

Mathematics, 18.10.2020 23:01

Mathematics, 18.10.2020 23:01



