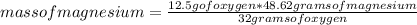Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Show how the limiting reactant is determined for 3.80 g of magnesium reacting with 12.5 g of oxygen....
Questions


Business, 10.12.2020 21:50


Mathematics, 10.12.2020 21:50

Health, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50

Biology, 10.12.2020 21:50


Mathematics, 10.12.2020 21:50

Physics, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50



Spanish, 10.12.2020 21:50


Spanish, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50


History, 10.12.2020 21:50