
Chemistry, 24.02.2021 21:30 CameronVand21
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?
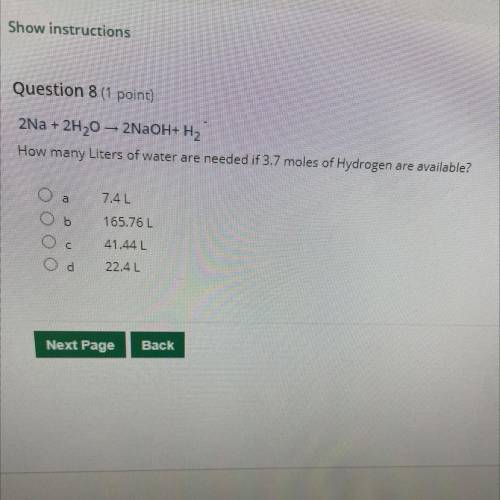

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions


English, 28.08.2019 14:20

Biology, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20

English, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20


Computers and Technology, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20



English, 28.08.2019 14:20

Mathematics, 28.08.2019 14:20


History, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20


Mathematics, 28.08.2019 14:20



