Percent of C-14 remaining in sample:
50%
Half-life of C-14:
5,730
Age estimate in...

Chemistry, 24.02.2021 23:40 emilybrown21304
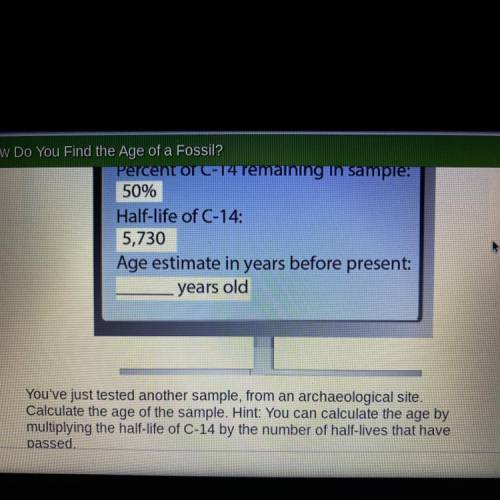
Percent of C-14 remaining in sample:
50%
Half-life of C-14:
5,730
Age estimate in years before present:
years old
You've just tested another sample, from an archaeological site.
Calculate the age of the sample. Hint: You can calculate the age by
multiplying the half-life of C-14 by the number of half-lives that have passed

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

You know the right answer?
Questions

Mathematics, 18.03.2020 04:33



Biology, 18.03.2020 04:34

Chemistry, 18.03.2020 04:34




Mathematics, 18.03.2020 04:34

Mathematics, 18.03.2020 04:34

World Languages, 18.03.2020 04:34

History, 18.03.2020 04:34



Mathematics, 18.03.2020 04:34

Mathematics, 18.03.2020 04:35







