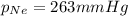A 2.00L mixture of helium, nitrogen, and neon has a total pressure of 815 mmHg at a
temperature of 255K. If the partial pressure of helium is 201 mmHg and the partial
pressure of nitrogen is 351 mmHg, what is the partial pressure of neon in the mixture?
O 709 mmHg
O 512 mmHg
O 667 mmHg
O 263 mmHg

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A 2.00L mixture of helium, nitrogen, and neon has a total pressure of 815 mmHg at a
temperature of...
Questions





Biology, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00






Computers and Technology, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00

Computers and Technology, 24.06.2019 23:00



Mathematics, 24.06.2019 23:00



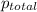 =total pressure of gases = 815 mm Hg
=total pressure of gases = 815 mm Hg
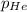 = partial pressure of helium = 201 mm Hg
= partial pressure of helium = 201 mm Hg
 = partial pressure of nitrogen = 351 mm Hg
= partial pressure of nitrogen = 351 mm Hg
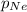 = partial pressure of Neon = ?
= partial pressure of Neon = ?