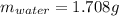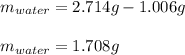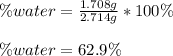A 2.714 gram sample of a hydrate of sodium carbonate is placed in a test
tube and heated until the mass is constant. If the mass of the dry sodium
carbonate is 1.006 grams. What was the mass of water driven off and what
was the percentage of water in the hydrate?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
A 2.714 gram sample of a hydrate of sodium carbonate is placed in a test
tube and heated until the...
Questions

Mathematics, 23.04.2020 22:56


Mathematics, 23.04.2020 22:56

Chemistry, 23.04.2020 22:56


Mathematics, 23.04.2020 22:56

Mathematics, 23.04.2020 22:56


Mathematics, 23.04.2020 22:56

Mathematics, 23.04.2020 22:56


Physics, 23.04.2020 22:57


Biology, 23.04.2020 22:57

Mathematics, 23.04.2020 22:57


Mathematics, 23.04.2020 22:57


Mathematics, 23.04.2020 22:57