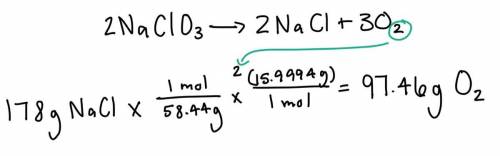Chemistry, 25.02.2021 09:00 komari0217
Given the following equation: 2NaClO3 --> 2NaCl + 3O2 If 178 g of NaCl are produced in this reaction, how many grams of O2 are also produced?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
Given the following equation: 2NaClO3 --> 2NaCl + 3O2
If 178 g of NaCl are produced in this reac...
Questions

Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Social Studies, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

English, 18.03.2021 02:10




Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10


Mathematics, 18.03.2021 02:10


Chemistry, 18.03.2021 02:10



History, 18.03.2021 02:10