
Chemistry, 25.02.2021 14:00 Dreambig85
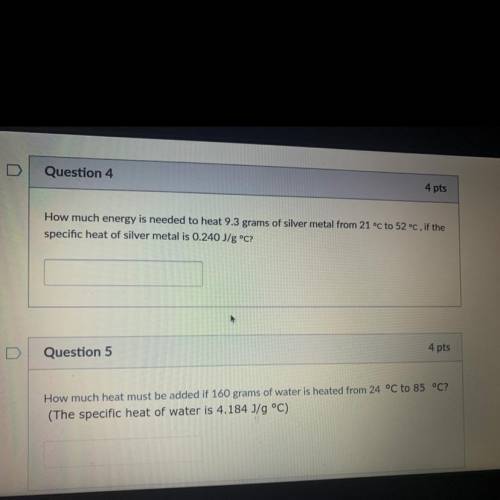
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific heat of silver metal is 0.240 J/g °C?
Question 5
4 pts
How much heat must be added if 160 grams of water is heated from 24 °C to 85 °C?
(The specific heat of water is 4.184 J/g °C)
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific he...
Questions

Health, 10.03.2020 23:39

Computers and Technology, 10.03.2020 23:39











History, 10.03.2020 23:39






English, 10.03.2020 23:40



