Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2
You know the right answer?
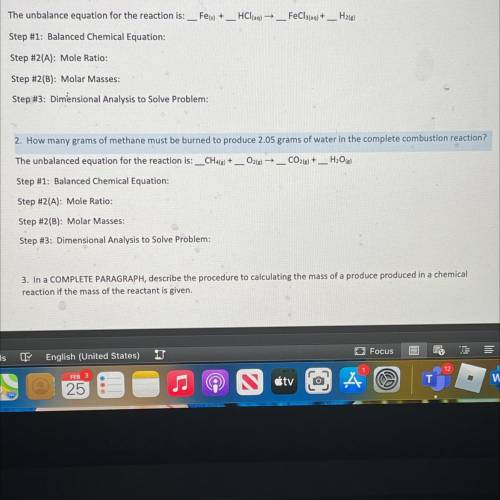
2. How many grams of methane must be burned to produce 2.05 grams of water in the complete combustio...
Questions

Mathematics, 18.05.2021 06:10





Biology, 18.05.2021 06:10


Mathematics, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20

Chemistry, 18.05.2021 06:20


Biology, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20

History, 18.05.2021 06:20


English, 18.05.2021 06:20

Mathematics, 18.05.2021 06:20