
Chemistry, 25.02.2021 18:40 fluffyanimal456
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation below: Cu + 2AgNO3 →Cu(NO3)2 + 2Ag Calculate the number of moles of copper(II) nitrate produced when 5 moles of copper react. Type your answer as a number with 1 significant figure. Make sure to include the correct units in your answer. Units are a type of measurement i. e. gram (g) or mole (mol). Do not include the chemical formula in your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Copper reacts with silver nitrate to produce silver and copper(II) nitrate according to the equation...
Questions

Mathematics, 09.10.2019 17:30





Mathematics, 09.10.2019 17:30

Computers and Technology, 09.10.2019 17:30



Mathematics, 09.10.2019 17:30




Mathematics, 09.10.2019 17:30

Computers and Technology, 09.10.2019 17:30


English, 09.10.2019 17:30

Geography, 09.10.2019 17:30

Computers and Technology, 09.10.2019 17:30

History, 09.10.2019 17:30

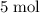 of
of 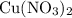 would be produced (assuming that reaction does not run out of
would be produced (assuming that reaction does not run out of 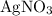 until all the
until all the 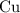 was converted.)
was converted.) 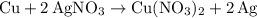 .
. . In other words, the actual equation for this reaction should be:
. In other words, the actual equation for this reaction should be: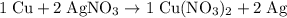 .
. .
. before running out of any other reactant.) This coefficient ratio would be equal to the ratio between:
before running out of any other reactant.) This coefficient ratio would be equal to the ratio between: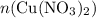 , the number of moles of
, the number of moles of 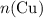 , the number of moles of
, the number of moles of 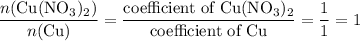 .
.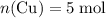 . Assume that
. Assume that 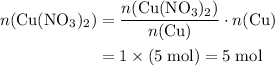 .
.

