
Chemistry, 25.02.2021 19:20 tyairamifflin2411
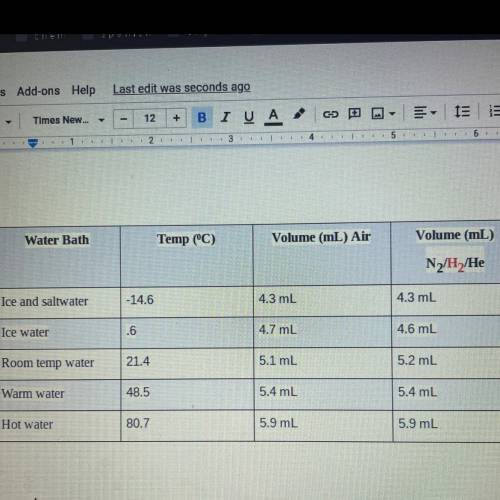
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula below
to determine your percent error for both gas samples.
Jexperimental value - actual valuel x 100
actual value
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in
each syringe? (Hint: Choose one volume and temperature pair from your data table to use
in your ideal gas law calculation.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Calculations:
1. The actual value for absolute zero in degrees Celsius is –273.15. Use the formula...
Questions

Mathematics, 16.03.2020 19:29






Biology, 16.03.2020 19:29


Social Studies, 16.03.2020 19:29




Mathematics, 16.03.2020 19:29


English, 16.03.2020 19:29


Mathematics, 16.03.2020 19:29


English, 16.03.2020 19:29

Chemistry, 16.03.2020 19:29



