

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The compound [Fe(SCN)(OH2 )5 ] 2 can be detected in the reaction of [Co(NCS)(NH3 )5 ]2 with Fe2 (aq)...
Questions

Mathematics, 14.12.2020 20:20


Mathematics, 14.12.2020 20:20


Mathematics, 14.12.2020 20:20





Mathematics, 14.12.2020 20:20

History, 14.12.2020 20:20




English, 14.12.2020 20:20

Mathematics, 14.12.2020 20:20

Mathematics, 14.12.2020 20:20



English, 14.12.2020 20:20

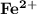 since it is an oxidizing agent that produces
since it is an oxidizing agent that produces  complex in solution.
complex in solution.![\mathbf{[Co(NH_3)_5(NCS)]^{+2}+[Fe(H_2O)_6]^{+2} \to [Fe(SCN) (H_2O)_5]^{+2}+ [Co(H_2O)_6]^{+2}}](/tpl/images/1147/8875/ef449.png)


