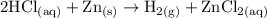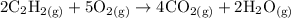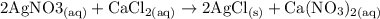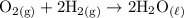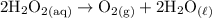Please help
Write a balanced equation for each chemical reactions. Include appropriate symbols. First reactant formula is given.
Hydrochloric acid (HCl) reacts with Zinc to form hydrogen gas and zinc chloride.
2 HCl +
Acetylene gas (C2H2) burns in a welding torch with oxygen to form carbon dioxide gas and water vapor. 2 C2H2 (g) +
c. Silver nitrate plus calcium chloride (CaCll2) yields silver chloride and calcium nitrate. 2 AgNO3 +
d. Oxygen gas combines with hydrogen gas to produce liquid water. O2 +
e. Hydrogen peroxide decomposes into oxygen gas and water when it contacts blood. 2 H2O2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 22:30
How might deforestation of a forest habitat directly affect populations of tree-dwelling animals?
Answers: 2
You know the right answer?
Please help
Write a balanced equation for each chemical reactions. Include appropriate symbols. Fir...
Questions



Mathematics, 07.07.2019 16:30

History, 07.07.2019 16:30

Social Studies, 07.07.2019 16:30





History, 07.07.2019 16:30

History, 07.07.2019 16:30







Social Studies, 07.07.2019 16:40

Health, 07.07.2019 16:40