Question 1 (1 point)
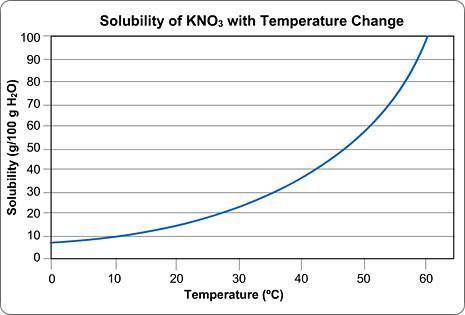
Above is a solubility curve for KNO3.
Solubility has nothing to do...

Chemistry, 26.02.2021 02:00 hectorgonzalejr333
Question 1 (1 point)
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it's a measure of how much salt will dissolve at a given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts of KNO3 in 100 g of water.
Approximately how many grams of KNO3 will dissolve in 100 g water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.
Question 1 options:
Question 2 (1 point)
Approximately what is the minimum temperature needed to dissolve 10 g of KNO3 in 100 g H2O?
Round your answer to the nearest whole number and type in the number only; no units.
Question 2 options:
Question 3 (1 point)
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.
Question 3 options:
Question 4 (1 point)
Saved
Increasing the temperature of the water will increase the amount of salt that it can dissolve.
Question 4 options:
True
False
Question 5 (1 point)
Saved
A solubility curve identifies how fast a solute will dissolve at different temperatures.
Question 5 options:
True
False
Question 6 (1 point)
Saved
This solubility curve indicates that at higher temperatures KNO3 will dissolve faster.
Question 6 options:
True
False
Question 7 (1 point)
Saved
A solubility curve tells you the minimum temperature needed to dissolve an amount of solute.
Question 7 options:
True
False

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Questions

Mathematics, 31.10.2020 01:20

English, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20



Mathematics, 31.10.2020 01:20

Computers and Technology, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20

English, 31.10.2020 01:20

Health, 31.10.2020 01:20

Mathematics, 31.10.2020 01:20

English, 31.10.2020 01:20

Physics, 31.10.2020 01:20


Physics, 31.10.2020 01:20


Mathematics, 31.10.2020 01:20

Chemistry, 31.10.2020 01:20

Chemistry, 31.10.2020 01:20



