
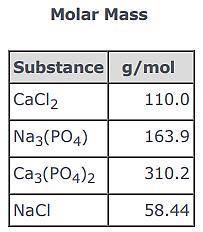
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the following question.
Suppose 163.9 g of Na3(PO4) reacted with sufficient CaCl2 in solution to actually yield 116 g of Ca3(PO4)2(s) . What is the percent yield of Ca3(PO4)2(s)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass...
Questions





Computers and Technology, 10.12.2019 21:31

















