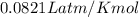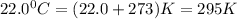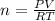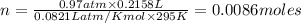Chemistry, 26.02.2021 02:20 jdjdjdjdjjffi7273
2) A student performs this reaction in the laboratory, and collects the hydrogen gas over water. The student collects 215.8 mL of gas. The total pressure is 755.2 mmHg and the temperature is 22.0 oC. c) Use the ideal gas law to calculate how many moles of hydrogen were produced in the reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
2) A student performs this reaction in the laboratory, and collects the hydrogen gas over water. The...
Questions

Social Studies, 25.02.2021 18:50

Biology, 25.02.2021 18:50


Computers and Technology, 25.02.2021 18:50

English, 25.02.2021 18:50



Social Studies, 25.02.2021 18:50


Mathematics, 25.02.2021 18:50

History, 25.02.2021 18:50



Mathematics, 25.02.2021 18:50




Mathematics, 25.02.2021 18:50

Mathematics, 25.02.2021 18:50

Arts, 25.02.2021 18:50

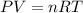
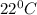 = 19.8 mm Hg
= 19.8 mm Hg