
An equilibrium mixture in a 16.2 L container at 712 K contains 0.218 mol NH4I(s), 0.189 M NH3 and 0.116 M HI. What will be the concentrations of the two gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 6.79 L

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1


Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
An equilibrium mixture in a 16.2 L container at 712 K contains 0.218 mol NH4I(s), 0.189 M NH3 and 0....
Questions


Mathematics, 29.11.2019 02:31


Chemistry, 29.11.2019 02:31


History, 29.11.2019 02:31

Mathematics, 29.11.2019 02:31


Arts, 29.11.2019 02:31

Social Studies, 29.11.2019 02:31

Geography, 29.11.2019 02:31


Physics, 29.11.2019 02:31



Mathematics, 29.11.2019 02:31


Physics, 29.11.2019 02:31


Mathematics, 29.11.2019 02:31

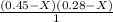 ----- ( 1 )
----- ( 1 ) 

