
Chemistry, 26.02.2021 04:40 gabrielleteti
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction?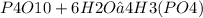

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction...
Questions

Mathematics, 05.06.2020 18:58

History, 05.06.2020 18:58

Law, 05.06.2020 18:58

Biology, 05.06.2020 18:58


Mathematics, 05.06.2020 18:58




SAT, 05.06.2020 18:58

History, 05.06.2020 18:58


Mathematics, 05.06.2020 18:58


Chemistry, 05.06.2020 18:58

Computers and Technology, 05.06.2020 18:58




Mathematics, 05.06.2020 18:58



