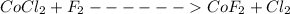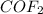Given the following unbalanced equation:
CoCl2 + F2 à CoF2 + Cl2
If you are given 11.84...

Chemistry, 26.02.2021 09:10 alexisthegirl
Given the following unbalanced equation:
CoCl2 + F2 à CoF2 + Cl2
If you are given 11.84 mole of CoCl2, how many moles of CoF2 will be produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Questions


Chemistry, 06.05.2021 20:10

Mathematics, 06.05.2021 20:10


English, 06.05.2021 20:10


Mathematics, 06.05.2021 20:10






Mathematics, 06.05.2021 20:10


History, 06.05.2021 20:10

Health, 06.05.2021 20:10



Mathematics, 06.05.2021 20:10