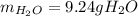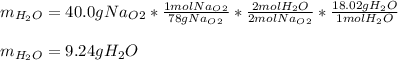A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O)...

Chemistry, 26.02.2021 09:20 milkshakegrande101
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
Questions

Mathematics, 28.04.2021 03:10


Biology, 28.04.2021 03:10


Mathematics, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10


Mathematics, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10

Computers and Technology, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10

Social Studies, 28.04.2021 03:10


English, 28.04.2021 03:10

Mathematics, 28.04.2021 03:10