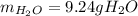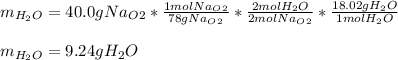A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O)...

Chemistry, 26.02.2021 09:20 jagslovegirl
A) What mass in grams of H20 is needed to react completely with 40.0 g of
Na2O2?
M(H2O) =18.02g/mol
M(NA2O2)= 78g/mol
Ecuation:
2Na2O2 (s)+2h2O(I)—> 4NaOH(aq) + O2 (g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Questions

Social Studies, 29.11.2021 23:50



Biology, 29.11.2021 23:50

Health, 29.11.2021 23:50

History, 29.11.2021 23:50


Mathematics, 29.11.2021 23:50

Chemistry, 29.11.2021 23:50






English, 29.11.2021 23:50





English, 29.11.2021 23:50