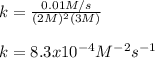Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
What is the rate constant of a reaction if rate = 1 x 10^-2 (mol/L)/s, [A] is 2 M,
[B] is 3 M, m =...
Questions


Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Physics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01




Mathematics, 20.09.2020 03:01



History, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01



Mathematics, 20.09.2020 03:01

![k=\frac{r}{[A]^2[B]}](/tpl/images/1150/6049/0baf7.png)