
Chemistry, 26.02.2021 14:00 daniel9299
A sample of Ar(g) is contained in a
cylinder with a moveable piston at an initial
temperature of T. The volume of the
sample is decreased from 4.5 L to 1.5 L
while the pressure is held constant, as
shown in the diagram below.


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
A sample of Ar(g) is contained in a
cylinder with a moveable piston at an initial
temperature...
temperature...
Questions

Physics, 28.02.2020 19:02




English, 28.02.2020 19:02

Mathematics, 28.02.2020 19:02

Mathematics, 28.02.2020 19:02







Mathematics, 28.02.2020 19:02






SAT, 28.02.2020 19:02

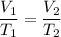
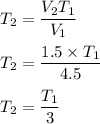
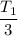 . Hence, the correct option is (B).
. Hence, the correct option is (B).

