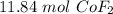Given the following unbalanced equation:
CoCl2 + F2 à CoF2 + Cl2
If you are given...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Questions

History, 09.07.2019 13:50

History, 09.07.2019 13:50

History, 09.07.2019 13:50

Social Studies, 09.07.2019 13:50

History, 09.07.2019 13:50

Biology, 09.07.2019 13:50

History, 09.07.2019 13:50

Mathematics, 09.07.2019 13:50

Physics, 09.07.2019 13:50

Social Studies, 09.07.2019 13:50

Biology, 09.07.2019 13:50

Mathematics, 09.07.2019 13:50



Advanced Placement (AP), 09.07.2019 13:50

Mathematics, 09.07.2019 13:50


Mathematics, 09.07.2019 13:50


Mathematics, 09.07.2019 13:50

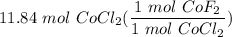 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: