
Chemistry, 27.02.2021 08:10 chunkymonkey090
PLEASE HELP WILL MARK BRAINLIEST + 40 POINTS
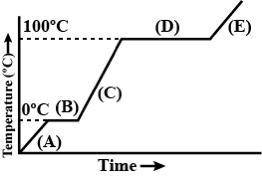
1. in which section of the graph does the substance exist as a liquid?
2.in which section of the graph does the substance exist as a gas?
3. in which section of the graph does the substance exist as a solid?
4. in which section of the graph is melting occurring?
5. in which section of the graph is vaporization occurring?
6. what is the boiling point of this substance?
7. what is the freezing point of this substance?
8. what is the meeting point of this substance?
9. which parts of the graph show a change in kinetic energy? (can be more than 1)
10. which sections of the graph show potential energy? (can be more than 1)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

You know the right answer?
PLEASE HELP WILL MARK BRAINLIEST + 40 POINTS
1. in which section of the graph does the substance ex...
Questions

Biology, 26.03.2020 23:02

Mathematics, 26.03.2020 23:02

Mathematics, 26.03.2020 23:02




Mathematics, 26.03.2020 23:03


Biology, 26.03.2020 23:03


Physics, 26.03.2020 23:03




Mathematics, 26.03.2020 23:03

Geography, 26.03.2020 23:03


Mathematics, 26.03.2020 23:03




