
Chemistry, 27.02.2021 14:10 kris22elizondop9v1bb
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸
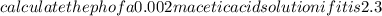

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸...
Questions


Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00



Health, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00





Mathematics, 18.12.2020 01:00


Chemistry, 18.12.2020 01:00




Mathematics, 18.12.2020 01:00



