Chemistry, 28.02.2021 17:20 lailabirdiemae
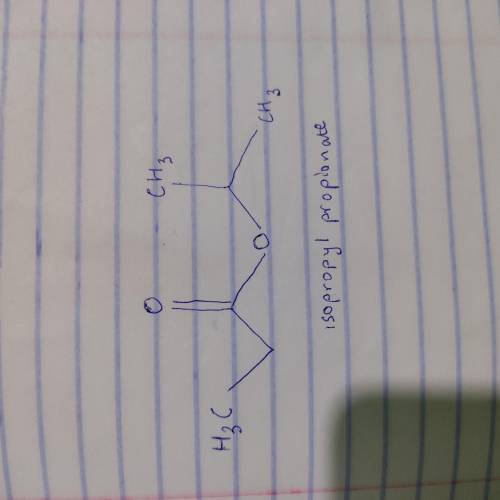
An unknown compound X has the empirical formula C3H6O and a molecular ion peak in its mass spectrum at m/z 116. X shows no IR absorption at 3200-3600 cm^-1 but shows a peak at 1740 cm^-1. The H NMR spectral data of X is shown below. What is the most likely structure of X? 22. An unknown compound X has the molecular formula C6H14O. X shows a strong peak in its IR spectrum at 3000 cm^-1. The 1H NMR spectral data of N are given below. What is the most likely structure of X?
Absorption ζ H ratio
triplet 1.0 3
double 1.0 6
quartet 2.0 2
septet 3.5 1

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
An unknown compound X has the empirical formula C3H6O and a molecular ion peak in its mass spectrum...
Questions

Mathematics, 25.07.2019 19:50

Spanish, 25.07.2019 19:50




Mathematics, 25.07.2019 19:50


Spanish, 25.07.2019 19:50




Mathematics, 25.07.2019 19:50

History, 25.07.2019 19:50


Mathematics, 25.07.2019 19:50

Chemistry, 25.07.2019 19:50


Biology, 25.07.2019 19:50

Chemistry, 25.07.2019 19:50

History, 25.07.2019 20:00