
Chemistry, 28.02.2021 19:40 heavenwagner
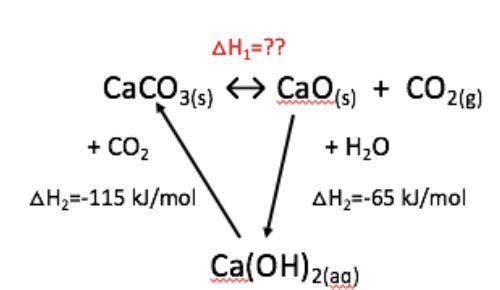
In this exercise you are asked to calculate the enthalpy change of lime burning, using the enthalpy values of the intermediary reactions. The Hess cycle diagram of the limestone cycle is enclosed for assistance.
CaCO3 ↔︎ CaO + CO2 △H1 = ???
CaO + H2O = Ca(OH)2 △H2 = -65 kJ/mol
Ca(OH)2 + CO2 = CaCO3 + H2O △H3 = -115 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
In this exercise you are asked to calculate the enthalpy change of lime burning, using the enthalpy...
Questions




Mathematics, 27.01.2021 16:30






Mathematics, 27.01.2021 16:30




Physics, 27.01.2021 16:30



Mathematics, 27.01.2021 16:30

Social Studies, 27.01.2021 16:30

Chemistry, 27.01.2021 16:30

Mathematics, 27.01.2021 16:30



