
Chemistry, 28.02.2021 21:10 cicimarie2018
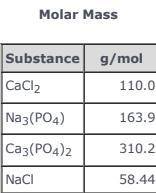
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass table above to answer the following question. Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl2 in solution yields 116 g ofCa3(PO4)2(s). What is the percent yield of Ca3(PO4)2(s)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s)+ 6NaCl(aq) Use the balanced equation and the Molar Mass ta...
Questions

Mathematics, 24.02.2021 02:30

Social Studies, 24.02.2021 02:30

German, 24.02.2021 02:30

English, 24.02.2021 02:30


Mathematics, 24.02.2021 02:30

Mathematics, 24.02.2021 02:30






Mathematics, 24.02.2021 02:30



Mathematics, 24.02.2021 02:30


Computers and Technology, 24.02.2021 02:30

English, 24.02.2021 02:30



