Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
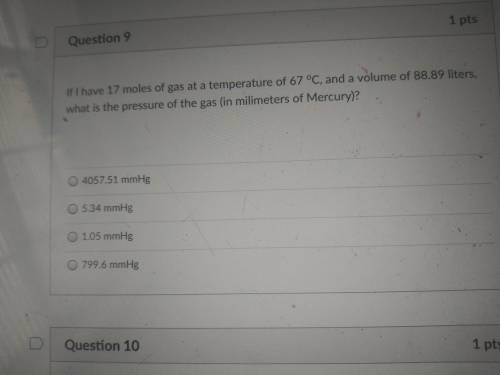
If i have 17 moles of gas at a temperature of 67 °C, and a volume of 88.89 liters, what is the press...
Questions






Mathematics, 08.01.2020 12:31





History, 08.01.2020 12:31

Mathematics, 08.01.2020 12:31

Mathematics, 08.01.2020 12:31


Chemistry, 08.01.2020 12:31



Mathematics, 08.01.2020 12:31

Physics, 08.01.2020 12:31