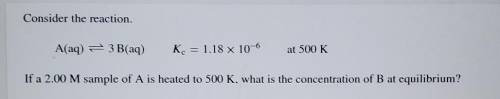Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
Consider the reaction. A(aq) = 3 B(aq) K. = 1.18 x 10^-6 at 500K If a 2.00 M sample of A is heated t...
Questions

History, 27.01.2020 18:31

English, 27.01.2020 18:31

History, 27.01.2020 18:31

History, 27.01.2020 18:31


English, 27.01.2020 18:31

History, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31


English, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31





Spanish, 27.01.2020 18:31

Chemistry, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31