
Chemistry, 01.03.2021 04:10 krystalhurst97
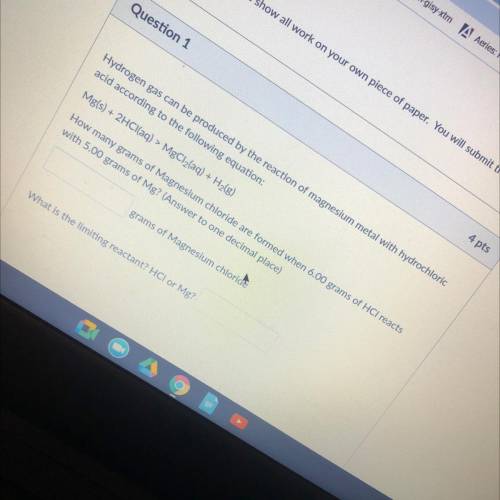
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are formed when 6.00 grams of HCl reacts
with 5.00 grams of Mg? (Answer to one decimal place)
grams of Magnesium chloride
What is the limiting reactant? HCI or Mg?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Hydrogen gas can be produced by the reaction of magnesium metal with hydrochloric
acid according to...
Questions

English, 11.07.2019 12:30


Mathematics, 11.07.2019 12:30

Biology, 11.07.2019 12:30

Mathematics, 11.07.2019 12:30

Social Studies, 11.07.2019 12:30

Social Studies, 11.07.2019 12:30


Mathematics, 11.07.2019 12:30

Mathematics, 11.07.2019 12:30


Mathematics, 11.07.2019 12:30

English, 11.07.2019 12:30

Social Studies, 11.07.2019 12:30

Chemistry, 11.07.2019 12:30

Social Studies, 11.07.2019 12:30

Social Studies, 11.07.2019 12:30



Social Studies, 11.07.2019 12:30



