Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
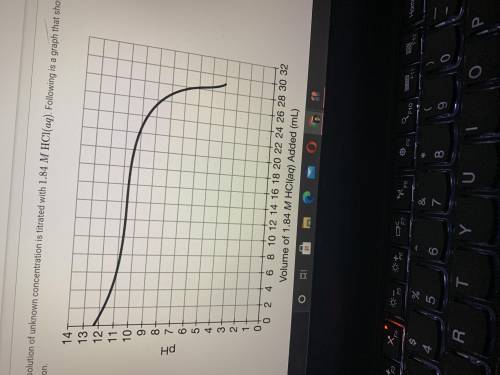
If 28.25mL of 1.84MHCl(aq) was required to reach the equivalence point, calculate the concentration...
Questions


Advanced Placement (AP), 10.09.2020 04:01


Computers and Technology, 10.09.2020 04:01

Computers and Technology, 10.09.2020 04:01

History, 10.09.2020 04:01

History, 10.09.2020 04:01








Biology, 10.09.2020 04:01




Mathematics, 10.09.2020 04:01