
Chemistry, 01.03.2021 18:30 Matseleng3775
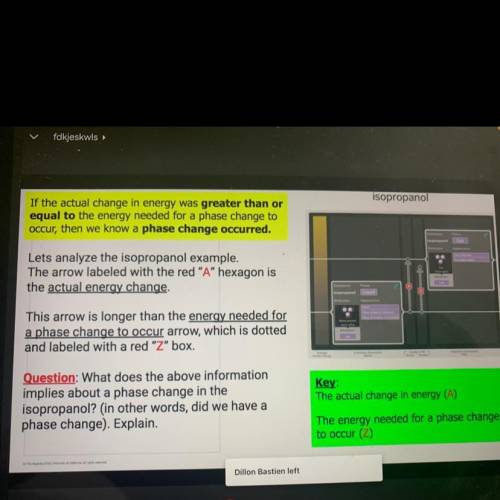
If the actual change in energy was greater than o
equal to the energy needed for a phase change to
occur, then we know a phase change occurred.
Lets analyze the isopropanol example,
The arrow labeled with the red "A" hexagon is
the actual energy change.
This arrow is longer than the energy needed for
a phase change to occur arrow, which is dotted
and labeled with a red "Z" box.
Question: What does the above information
implies about a phase change in the
isopropanol? (in other words, did we have a
phase change). Explain.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
If the actual change in energy was greater than o
equal to the energy needed for a phase change to<...
Questions


History, 03.10.2020 01:01



Mathematics, 03.10.2020 01:01





Mathematics, 03.10.2020 01:01




Engineering, 03.10.2020 01:01

Mathematics, 03.10.2020 01:01

Social Studies, 03.10.2020 01:01


Arts, 03.10.2020 01:01




