Choose whether the reaction is exothermic or endothermic.
2C(s) + 2H2(g) → C2H4(9) -Select--
...

Chemistry, 01.03.2021 20:00 godisgoodallthoubxi7
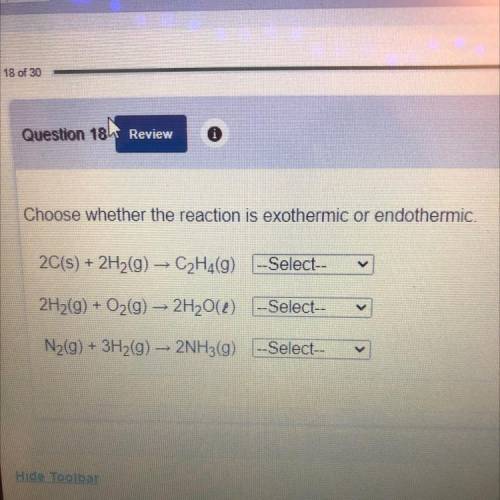
Choose whether the reaction is exothermic or endothermic.
2C(s) + 2H2(g) → C2H4(9) -Select--
2H2(g) + O2(g) → 2H20() -Select--
N2(g) + 3H2(g) + 2NH3(g) --Select--

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Questions


Mathematics, 25.10.2019 19:43





















