
Chemistry, 01.03.2021 21:50 lanaiheart7
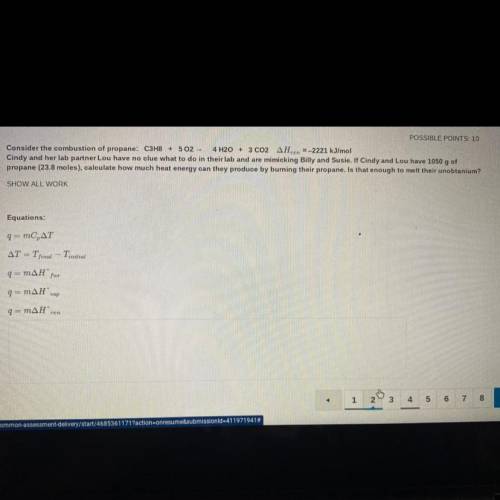
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab partner Lou have no clue what to do in their lab and are mimicking Billy and Susie. If Cindy and Lou have 1050 g of
propane (23.8 moles), calculate how much heat energy can they produce by burning their propane. Is that enough to melt their unobtanium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab...
Questions



Mathematics, 10.11.2020 02:30

Geography, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30


Mathematics, 10.11.2020 02:30

History, 10.11.2020 02:30



History, 10.11.2020 02:30



Spanish, 10.11.2020 02:30

English, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30




Mathematics, 10.11.2020 02:30



