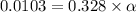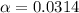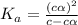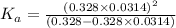Chemistry, 01.03.2021 22:20 william1690
In the laboratory, a general chemistry student measured the pH of a 0.328 M aqueous solution of acetylsalicylic acid (aspirin), HC9H7O4 to be 1.987. Use the information she obtained to determine the Ka for this acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3

Chemistry, 23.06.2019 14:00
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1

Chemistry, 23.06.2019 14:10
What is true according to the second law of thermodynamics
Answers: 1
You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.328 M aqueous solution of acet...
Questions

Mathematics, 01.11.2019 03:31


Mathematics, 01.11.2019 03:31

History, 01.11.2019 03:31





Business, 01.11.2019 03:31




Mathematics, 01.11.2019 03:31



Biology, 01.11.2019 03:31

Mathematics, 01.11.2019 03:31

Mathematics, 01.11.2019 03:31


History, 01.11.2019 03:31

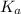 for the acid is
for the acid is 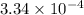
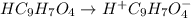
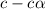
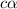
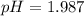
![1.987=-log[H^+]](/tpl/images/1158/1923/8f2fb.png)
![[H^+]=0.0103](/tpl/images/1158/1923/4a7f1.png)
![[H^+]=c\times \alpha](/tpl/images/1158/1923/4fc41.png)