
Chemistry, 01.03.2021 22:20 jacobp0712
In the lab activity, the reaction rate was determined by the appearance of a product. However, the reaction rate can also be determined by the disappearance of a reactant. Rate =Δ[product]/Δt or rate−Δ[reactant]Δ t. In each situation below, you are given a rate measured by the appearance of one component of the reaction and are asked to predict the rate of appearance or disappearance of another component, based on logic and stoichiometric relationships.
For example, if the reaction is as follows:
A+2B⟶products
For every mole of A that is used, 2 moles of B are used so the rate of disappearance of B is twice the rate of the disappearance of A.
This may be expressed as:
rate=−Δ[B]/Δt=−2[A]/Δt , N2(g)+3H2(g)⟶2NH3(g)
The reaction rate is measured as 0.032 M NH3/s. Determine the rate of disappearance of N2 and the rate of disappearance of H2. Explain how you arrived at your answers.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
In the lab activity, the reaction rate was determined by the appearance of a product. However, the r...
Questions


Business, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31


Mathematics, 10.01.2020 16:31

World Languages, 10.01.2020 16:31



Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31

Mathematics, 10.01.2020 16:31



Mathematics, 10.01.2020 16:31

History, 10.01.2020 16:31

History, 10.01.2020 16:31



Social Studies, 10.01.2020 16:31

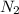 = 0.032 M/s
= 0.032 M/s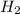 = 0.096 M/s
= 0.096 M/s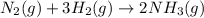
![-\frac{1d[N_2]}{dt}](/tpl/images/1158/2044/f2cf6.png)
![-\frac{1d[H_2]}{3dt}](/tpl/images/1158/2044/96c4e.png)
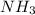 =
= ![\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/ceb35.png)
![-\frac{1d[N_2]}{dt}=-\frac{1d[H_2]}{3dt}=\frac{1d[NH_3]}{2dt}](/tpl/images/1158/2044/4e2ff.png)
![-\frac{d[H_2]}{dt}=3\times 0.032M/s=0.096M/s](/tpl/images/1158/2044/7af2b.png)



