
Chemistry, 02.03.2021 01:40 andrewjarrah05
Balance each redox reactions, Identify the entities reduced or oxidized. State the agents.
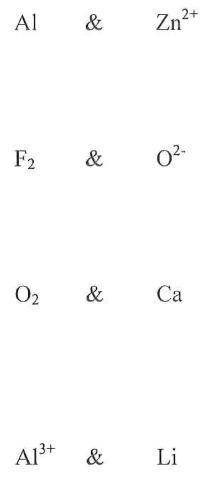
1. Al + Zn2+
2. F2 + O2-
3. O2 + Ca
4. Li + Al3+

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Balance each redox reactions, Identify the entities reduced or oxidized. State the agents.
1. Al +...
Questions

Mathematics, 24.10.2019 20:43












History, 24.10.2019 20:43

Mathematics, 24.10.2019 20:43


English, 24.10.2019 20:43




History, 24.10.2019 20:43



