Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
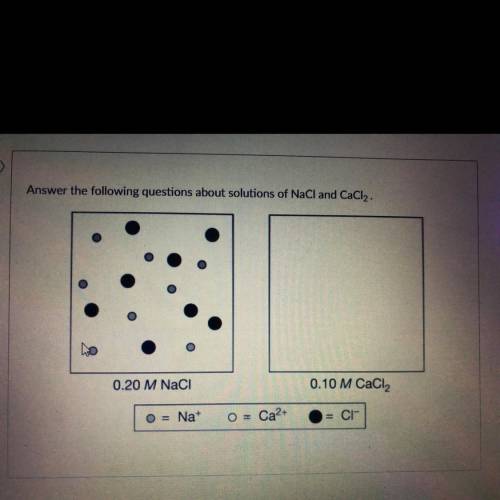
Describe the intermolecular forces present in each aqueous solution. Which solution would have a gre...
Questions


Physics, 21.04.2021 14:00


Mathematics, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00

Computers and Technology, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00


Mathematics, 21.04.2021 14:00

English, 21.04.2021 14:00


Mathematics, 21.04.2021 14:00

Chemistry, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00



Mathematics, 21.04.2021 14:00

Mathematics, 21.04.2021 14:00