
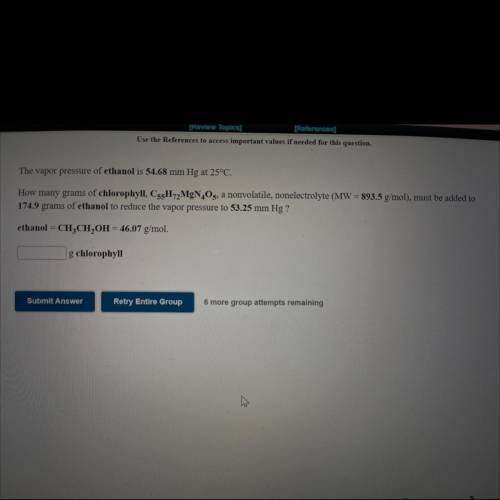
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5, a nonvolatile, nonelectrolyte (MW = 893.5 g/mol), must be added to
174.9 grams of ethanol to reduce the vapor pressure to 53.25 mm Hg ?
ethanol = CH3CH2OH = 46.07 g/mol.
How many ___g chlorophyll?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5,...
Questions


Mathematics, 19.11.2020 23:10


Physics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

World Languages, 19.11.2020 23:10


Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10




Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10

English, 19.11.2020 23:10


History, 19.11.2020 23:10


Mathematics, 19.11.2020 23:10

Mathematics, 19.11.2020 23:10



